Healthcare doesn’t move on a clean schedule. People don’t decide to quit smoking or start treatment after scrolling through three pages of brand messaging or watching a campaign video.
It happens in a moment, and that’s when people search for help. Maybe they’re looking for nicotine replacement therapy. Maybe they’re comparing treatment options. Either way, in that moment, they’re overwhelmed and need clarity.
But the confusion isn’t always about wrong or outdated clinical information. It’s baked into the systems behind the scenes, the operational drag that gets in the way of care.
We partnered with a multinational pharma company to solve a similar problem affecting their nicotine replacement therapy (NRT) products. They were managing product websites separately across Denmark, Finland, the Netherlands, Norway, Sweden, and Australia.
Here are the challenges they faced and how the right technology helped fix them.
Challenges
Duplicated systems slow teams down
Each country maintained its publishing platform. That means separate vendor contracts, hosting arrangements, feature development cycles, and support teams. When the same functionality needs to be rebuilt five or six times, it impacts security and performance.
Content management became disconnected
Without a central system, teams are left to copy content between markets. Campaigns were manually adapted, clinical updates had to be re-entered, and translations were handled outside the platform.
User experience became uneven
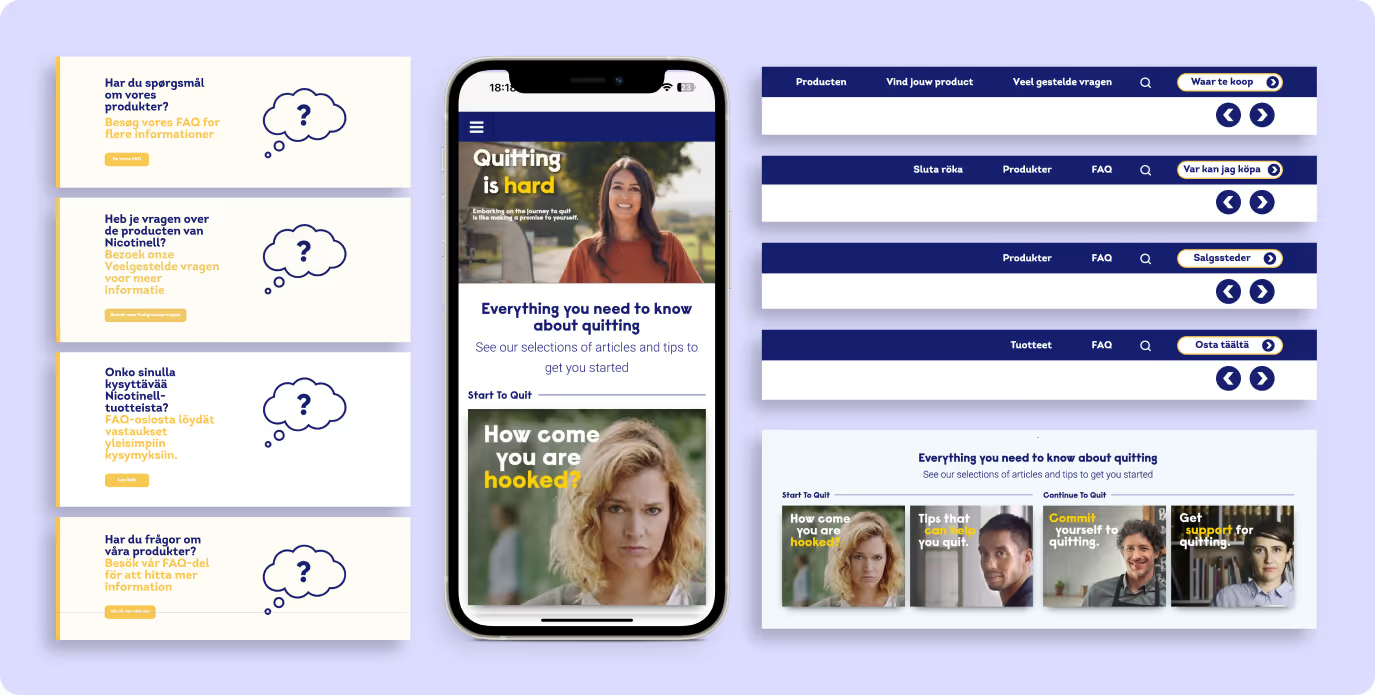
Users were not able to find the right product for their needs because the guidance or disclaimers differ by region. And if content isn’t in their native language, they’re less likely to take action.
Compliance is harder to track
Approval workflows and documentation standards vary across markets. When content is scattered across platforms, it's difficult to monitor what’s live, what’s approved, and what needs to be reviewed.
In therapeutic categories, this opens the door to regulatory gaps and reputational risk.
What we delivered and why it mattered
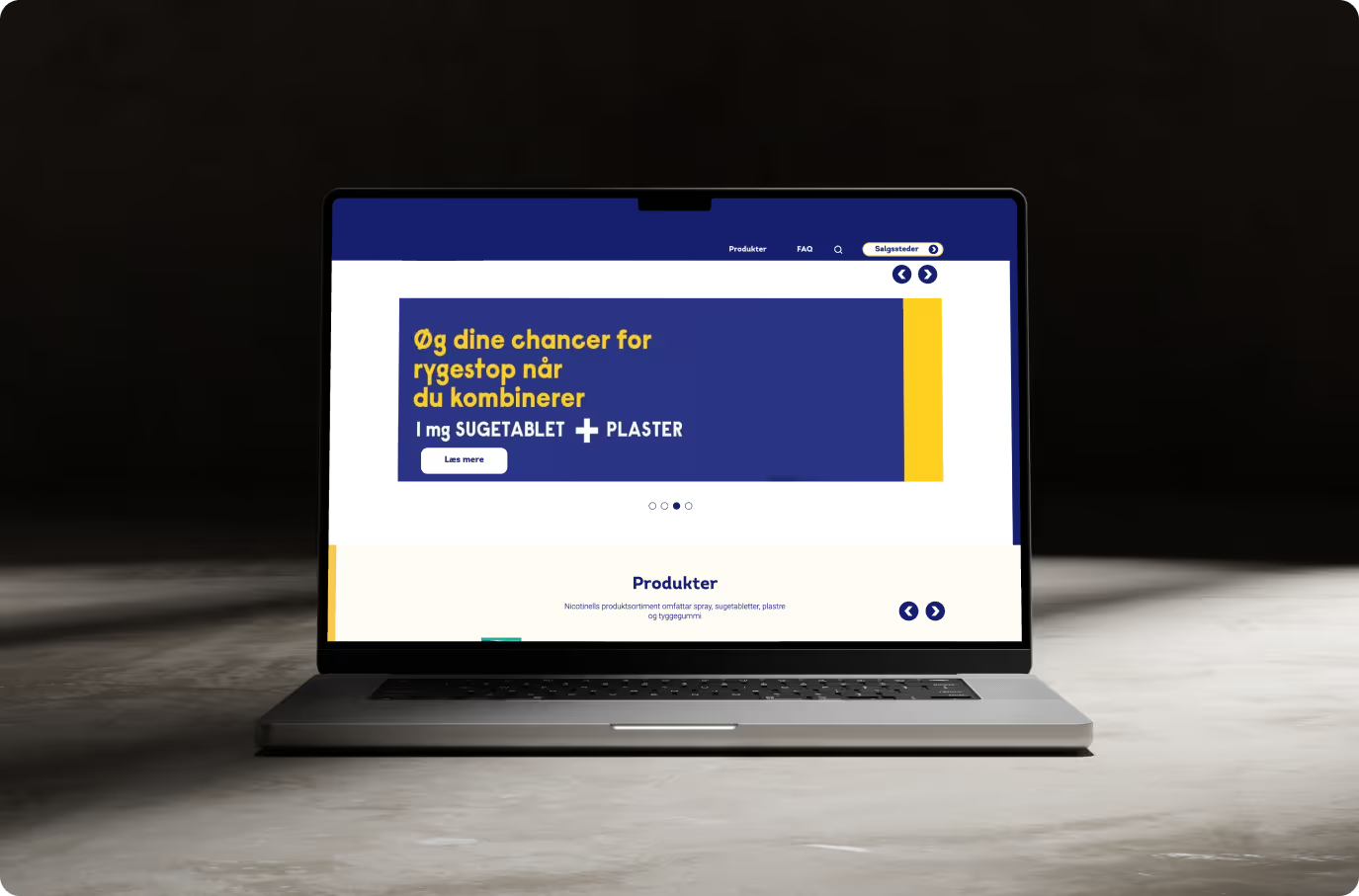
A component-based platform to eliminate rebuilds
Each market was rebuilding core features like product finders, dosage tools, and support content from scratch. We replaced that with a shared component library on Drupal. Features could be reused and configured locally. This cut down repetitive development, reduced cost, and ensured consistent functionality across markets. Local teams worked faster, and global teams had better control over platform standards.
A phased rollout based on actual system audits
We audited each market’s setup to understand what needed to be migrated, rebuilt, or removed. Rollouts were done in stages, with testing before launch. This avoided disruption and kept publishing operations running throughout the transition. Teams stayed productive, and adoption was faster because the system was built around their actual needs.
Scoped workflows aligned with local compliance
We created approval workflows and user roles to reflect how each country’s regulatory, medical, and legal processes worked. Teams followed their existing review structures, but with better tooling. This reduced manual tracking, improved turnaround times, and made it easier to stay audit-ready without extra effort.
Built-in localisation and translation tools
Translation workflows were embedded directly into the CMS. Local teams could manage language versions, metadata, and disclaimers without using external tools. This improved accuracy, reduced delays, and kept local content aligned with regulatory requirements.
Faster, more reliable platform performance
The platform was rebuilt to be lightweight, mobile-optimised, and easy to maintain. Load times improved across all regions. Publishing became more efficient, and support tickets dropped as common technical issues were removed at the source.
Shared analytics to track performance at every level
We implemented standard analytics across all markets. Teams could measure what content was published, how it performed, and where updates were needed. Everyone worked from the same data, without needing separate tools or reports.
Every implementation decision focused on reducing operational drag, removing redundant effort, improving time to market, and lowering long-term costs.
The result is a single platform that makes global content operations more efficient, more consistent, and easier to manage.

Conclusion
The right technology, tied to clear outcomes, helped solve key operational issues for the multinational pharma.
Solving the problem of operational drag isn’t unique to one company. It applies across healthcare, where systems should match the urgency of the decisions they support. When people search for help, it shouldn’t be seen as just another interaction or data point on a customer journey map. It’s a turning point, where their decision, mindset, or health outcome might shift direction. They need information they can trust, delivered quickly, in their language, and within their local regulatory context.
In the case of nicotine replacement therapy, smoking cessation, like many life-changing healthcare decisions, happens in minutes. The commitment is quick. People might take weeks to get there, but once they decide, they want to act immediately.
This is where disconnected digital systems fall short. They can’t keep up. People drop off. And the original intent to give people the right information and access to the product at the right time falls apart.
Motivation doesn’t wait. Anything inaccurate or slow became an expensive delay for both the pharma company and the people it’s meant to serve.

.avif)